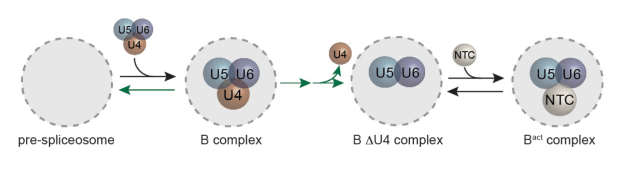
From Science at Brandeis:
“All animal and plant cells contain a highly elaborate system of filamentous protein polymers called the actin cytoskeleton, a scaffold that can be rapidly transformed to alter a cell’s shape and function. A critical step in reconfiguring this scaffold is the rapid disassembly (or turnover) of the actin filaments. But how is this achieved? It has long been known that the protein Cofilin plays a central role in this process, but it has been unclear how Cofilin achieves this feat. Cofilin can sever actin filaments into smaller fragments to promote their disassembly, but whether it also catalyzes subunit dissociation from filament ends has remained uncertain and controversial. Until now, this problem has been difficult to address because of limitations in directly observing Cofilin’s biochemical effects at filament ends….” Dr. Shashank Shekhar, working together with Dr. Johnson Chung and “jointly mentored by Bruce Goode, Jeff Gelles and Jane Kondev, use[d] microfluidics-assisted single molecule TIRF imaging to tackle the problem.
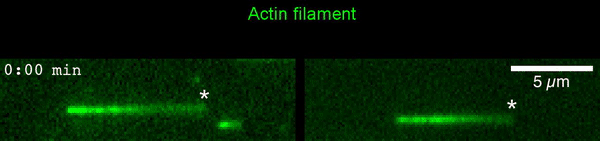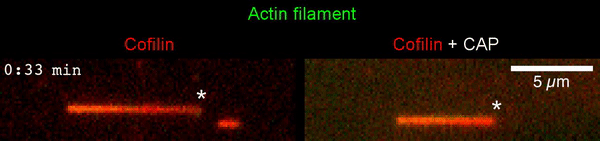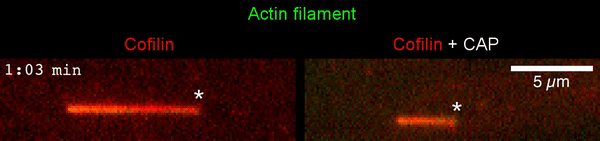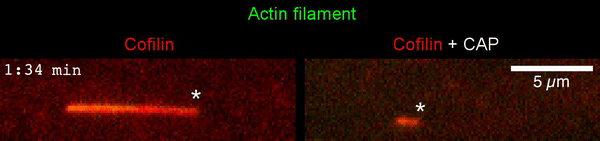 The new study shows that Cofilin and one other protein (Srv2/CAP) intimately collaborate at one end of the actin filament to accelerate subunit dissociation by over 300-fold! These are the fastest rates of actin depolymerization ever observed. Further, these results establish a new paradigm in which a protein that decorates filament sides (Cofilin) works in concert with a protein that binds to filament ends (Srv2/CAP) to produce an activity that is orders of magnitude stronger than the that of either protein alone.”
The new study shows that Cofilin and one other protein (Srv2/CAP) intimately collaborate at one end of the actin filament to accelerate subunit dissociation by over 300-fold! These are the fastest rates of actin depolymerization ever observed. Further, these results establish a new paradigm in which a protein that decorates filament sides (Cofilin) works in concert with a protein that binds to filament ends (Srv2/CAP) to produce an activity that is orders of magnitude stronger than the that of either protein alone.”
Shekhar S. et al. Synergy between cyclase-associated protein and cofilin accelerates actin filament depolymerization by two orders of magnitude.
Nature Communications 10, 5319 (2019).

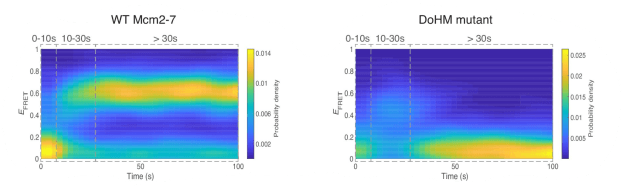
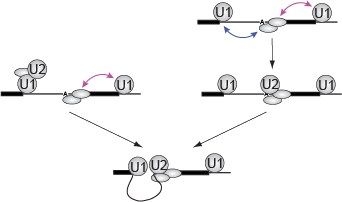
 Sarah Stumper and her collaborators used fluorescence correlation spectroscopy and multi-wavelength single-molecule fluorescence colocalization microscopy to show that the SCFs likely repress transcription through an interesting “delayed inhibition” mechanism in which the proteins arrive at DNA already complexed to RNA polymerase and block at a later stage of transcription initiation. The work explains factors that control the relative contributions of the two proteins to regulation and suggests a mechanism by which repression is restricted to ribosomal RNA and other promoters that form short-duration complexes with RNA polymerase.
Sarah Stumper and her collaborators used fluorescence correlation spectroscopy and multi-wavelength single-molecule fluorescence colocalization microscopy to show that the SCFs likely repress transcription through an interesting “delayed inhibition” mechanism in which the proteins arrive at DNA already complexed to RNA polymerase and block at a later stage of transcription initiation. The work explains factors that control the relative contributions of the two proteins to regulation and suggests a mechanism by which repression is restricted to ribosomal RNA and other promoters that form short-duration complexes with RNA polymerase.
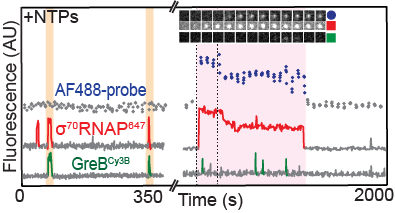
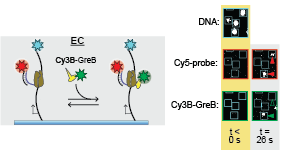 on elongation complex and modulate its activity. In this paper, Larry Tetone, Larry Friedman, and Melissa Osborne, along with their collaborators from the Gelles and
on elongation complex and modulate its activity. In this paper, Larry Tetone, Larry Friedman, and Melissa Osborne, along with their collaborators from the Gelles and