Grp94 is a molecular chaperone that helps to fold and maintain the folded state of “client” proteins in the endoplasmic reticulum. Acceleration of client folding is driven by conformational changes in Grp94. However, the sequence of conformational changes and how these changes are coupled to the cycle of ATP hydrolysis is not well understood. Prof. Timothy Street and his lab members Bin Huang and Ming Sun, in collaboration with Larry Friedman, did single-molecule fluorescence resonance energy transfer (FRET) experiments to directly observe conformational cycling in individual Grp94 molecules. Their studies show that ATP hydrolysis can drive repeated cycling between alternative “closed” states of Grp94, suggesting a way that enzyme might propagate structural changes to client molecules. 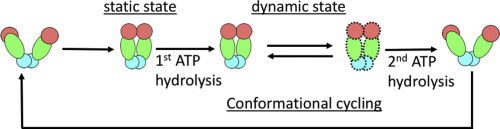
Conformational Cycling within the Closed State of Grp94, an Hsp90-Family Chaperone
Huang, B., Friedman, L.J., Gelles, J., Sun, M., and Street, T.O.
Journal of Molecular Biology 431, 3312-3323 (2019).


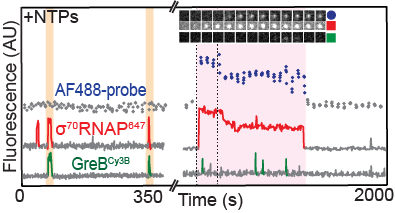 Sarah Stumper and her collaborators used fluorescence correlation spectroscopy and multi-wavelength single-molecule fluorescence colocalization microscopy to show that the SCFs likely repress transcription through an interesting “delayed inhibition” mechanism in which the proteins arrive at DNA already complexed to RNA polymerase and block at a later stage of transcription initiation. The work explains factors that control the relative contributions of the two proteins to regulation and suggests a mechanism by which repression is restricted to ribosomal RNA and other promoters that form short-duration complexes with RNA polymerase.
Sarah Stumper and her collaborators used fluorescence correlation spectroscopy and multi-wavelength single-molecule fluorescence colocalization microscopy to show that the SCFs likely repress transcription through an interesting “delayed inhibition” mechanism in which the proteins arrive at DNA already complexed to RNA polymerase and block at a later stage of transcription initiation. The work explains factors that control the relative contributions of the two proteins to regulation and suggests a mechanism by which repression is restricted to ribosomal RNA and other promoters that form short-duration complexes with RNA polymerase.